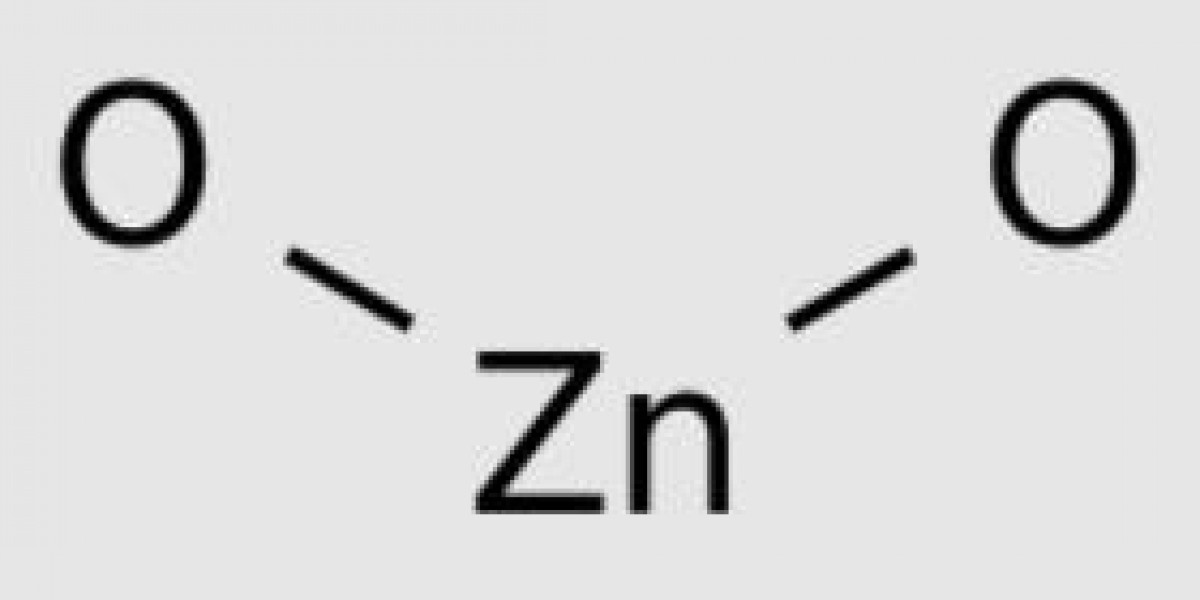
Properties of Zinc hydroxide (zinc hydroxide formula)
Physical properties of Zinc hydroxide:
The density of Zinc hydroxide is 3.05 g/cm3.
The Molar mass of Zinc hydroxide is 99.424 g/mol.
The melting point of Zinc hydroxide is 125 ° C (257 ° F).
The appearance is dull white flocculent sediment, odorless.
The valence of zinc in Zinc hydroxide is 2, and the Oxidation state is+2.
The pH of Zinc hydroxide is 8.88.
Zinc hydroxide is slightly soluble in water and insoluble in alcohol.
Chemical properties of Zinc hydroxide:
Aluminum reacts with Zinc hydroxide solution to form white precipitate, which dissolves in excess reagent to form Al (OH) 4 complex, indicating the existence of aluminum.
2Al3++(aq)+3Zn (OH) 2 (aq) → 2Al (OH) 3 (s)+3Zn
Zinc ion reacts with hydrogen sulfide in the presence of ammonia, and ammonium chloride forms white Zinc sulfide precipitate. Soluble in acids.
Zn2+(aq)+S2 → ZnS
Use of Zinc hydroxide
It is used as an adsorbent in medicine and as a fixative in dressings.
Zinc compounds are used in large dressings that absorb blood after surgery.
It is useful as an intermediate in the industrial processes of pesticides and pigments.
Sample questions
Question 1: Is Zinc hydroxide a salt?
Answer:
Under normal conditions, zinc salt dissociates to form zinc ions, and zinc ions combine with two sodium hydroxide ions in sodium hydroxide solution to form Zinc hydroxide. These properties of Zinc hydroxide are widely used to detect the presence of zinc ions in solutions.
Question 2: Is Zinc hydroxide soluble or insoluble?
Answer:
As we all know, Zinc hydroxide is slightly soluble in water and becomes more soluble with the decrease or increase of pH value.
Question 3: What is Zinc hydroxide composed of?
Answer:
Zinc chloride or Zinc sulfate reacts with sodium hydroxide to produce Zinc hydroxide. Zinc oxide cannot be dissolved with dilute sodium hydroxide solution.
Question 4: Is Zinc hydroxide acidic?
Answer:
Zinc hydroxide is a Inorganic compound with the chemical formula Zn (OH) 2. It is essentially bisexual and soluble in strong alkaline and acidic solutions.
Question 5: Why do we use zinc oxide as a pigment?
Answer:
One of the most important components is zinc oxide. Mainly used for primer and exterior wall paint. Zinc oxide has properties such as mold resistance, anti-corrosion, and stain resistance. Zinc oxide is a basic white pigment commonly known as zinc white or Chinese white.
Question 6: What happens when zinc reacts with dilute sulfuric acid?
Answer:
When dilute sulfuric acid is poured into zinc particles, zinc, which is more active than hydrogen, will replace it in acid to produce Zinc sulfate and hydrogen. Hydrogen is a combustible gas that emits a unique explosive sound when burned.